There’s been a lot of news about the Food and Drug Administration’s (FDA) changes to the Nutrition Facts label over the last couple years – but what does it mean? Whether you’re a buyer or reader of food labels, it would be beneficial to familiarize yourself with these anticipated updates so you can better understand the Nutrition Facts on food products. In this article, we will review some recent changes happening with nutrition facts labeling so you can rest assured that your food labels are FDA compliant.
Quick Background on the Nutrition Facts Label
Over 20 years ago in 1993, the FDA introduced the Nutrition Facts table to standardize how nutritional information was presented on packaged food and beverage products. The Nutrition Facts label was mandated after the passage of the Nutrition Labeling and Education Act of 1990. Before then, nutrition labeling was voluntary and varied from product to product. The United States’ inception of the Nutrition Facts table was the first of its kind and has since been adapted by other countries.
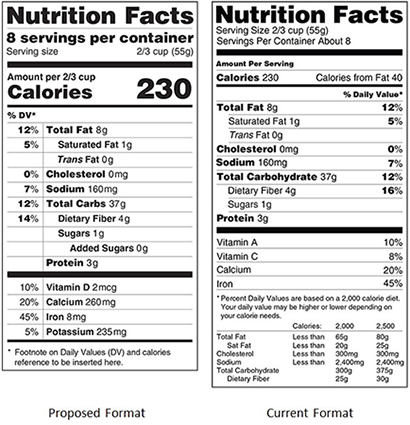
Changes Proposed to Nutrition Facts Label (March 2014)
On February 27, 2014, the FDA released its proposed updates for the new Nutrition Facts label. Several days later on March 3, the FDA began a 90-day public comment period to review feedback and address concerns over its proposed updates, shown below. The FDA is still formulating these changes but when finalized, manufacturers will have two years to get their labels compliant.
- Calories will become more prominent while ‘calories from fat’ will be eliminated.
- Differentiation of natural and added sugars.
- Serving sizes presented more realistically.
- Adjustments to some daily values, in particular, sodium.
Why the Updates?
One of the reasons behind the FDA’s proposed updates is due to the fact that the Nutrition Facts label has remained largely unchanged since the early 1990s. Although the updates are still being formulated, many health and nutrition organizations consider the changes as a step forward in the right direction for helping consumers make healthful decision.
According to the FDA’s website:
Paula Trumbo, Ph.D., acting director of FDA’s nutrition programs staff explains that updates are being assessed to address such factors as current nutrient recommendations, public health concerns based on recent data on food consumption, and the agency’s desire to make this information as clear and useful as possible.
Americans are paying more attention to food labels now than ever before whether to watch their calorie and sodium intake or to check for allergen ingredients for children. According to the FDA’s Health and Diet Survey conducted in 2002 and 2008 (most recent), the percentage of consumers who reported that they often use the label rose from 44 to 54 percent. The way Americans eat has changed since the Nutrition Facts label was first introduced and the FDA hopes to make food labels more reflective of current nutritional information and eating habits.
Added Sugars and Footnote (July 2015)
In July 2015, the FDA issued a proposed rule that would require a percent daily value (%DV) for added sugars, and changes to the current footnote on the Nutrition Facts label. This proposed rules would amend the FDA’s food labeling regulations to provide updated nutrition information on the Nutrition Facts and Supplement Facts labels to help consumers maintain healthy habits. The FDA accepted public comments on the supplemental proposed rule from July 27 to October 13. For more information, please visit the FDA’s “Proposed Changes to the Nutrition Facts Label” page.
Defining “Natural” on Food Labels (Nov. 2015)
Food products and consumer preferences have changed quite a bit since the original Nutrition Facts was created. One particular trend, organic and “free-from” foods, has led to questions about what “natural” means on food labels and how it should be defined. On Nov. 12, 2015, the FDA started accepting comments from the public on “natural” food labeling to answer such questions as: is it appropriate to define the term “natural”? If so, how should the FDA define “natural”? And how should the FDA determine the appropriate use of the term on food labels? Public comments on these questions will be accepted until May 10, 2016 so we still have a ways to go before anything is final. Learn more about “natural” food labels in our overview.
Although nothing has been officially changed with the Nutrition Facts label and “natural” term, we hope this information is helpful as you prepare for future compliance. Stay up to date with the latest labeling news by subscribing to our e-newsletter – we will continue to monitor any progress and updates so your operations can continue running smoothly.
Other Sources:
- Edney, Anna. “U.S. Food Labels to Get First Update by FDA in 21 Years.” Bloomberg. January 24, 2014. <http://www.bloomberg.com/news/2014-01-24/u-s-food-labels-to-get-first-update-by-fda-in-21-years.html>.
- “Food Labeling: Revision of the Nutrition and Supplement Facts Labels; Supplemental Proposed Rule To Solicit Comment on Limited Additional Provisions.” Federal Register. July 27, 2015. <http://www.federalregister.gov/articles/2015/07/27/2015-17928/food-labeling-revision-of-the-nutrition-and-supplement-facts-labels-supplemental-proposed-rule-to>
Editor’s Note: This post was originally published in February 2014 and has been updated for accuracy and comprehensiveness.