Introduction to FDA Nutrition Facts Labels
Editor’s Note: In 2016, the FDA finalized the new Nutrition Facts label. Manufacturers will have until July 26, 2018 to comply with the final requirements, and manufacturers with less than $10 million in annual food sales will have an additional year to make the changes. Learn more here.
Did you know that it is unlawful for your packaged food product to be without a nutrition fact label? Before you start marketing and selling your food product, you should make sure that your custom food label complies with the Food and Drug Administration (FDA) standards. We have compiled some information to help guide you through the process from chemical findings—to font—to finish!
Despite what most consumers believe, the FDA does not test the nutrition—the chemical breakdown—of every finished food or vitamin label on the shelf. In fact, submission of the nutritional data of your foodstuff to the FDA is completely voluntary. The FDA merely requires that the food be labeled with accurate nutritional facts, and how that table should appear on the product’s label. However, the rules for compliance can be very complex and must be followed carefully.
An example of an FDA regulated panel format:
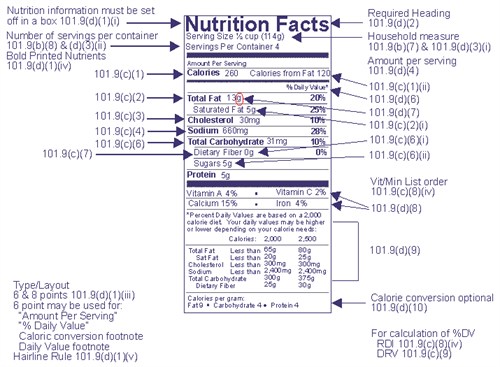
The FDA recommends laboratory analysis of each nutrient and recommends only one testing agency: the Association of Official Analytical Chemists International, a non-profit scientific association.
It’s not the responsibility of Consolidated Label, or any labeling company, to ensure that the Nutrition Facts panel ingredients are all valid and follow the FDA standard. The nutrition statements on your label need to be tested by AOAC, or another chemical analysis company, and the panel designed by the food manufacturer to reflect those findings.
Below are frequently asked questions and answers; they are intended solely as a guidance tool and not legal advisement.
The Essentials: Testing, Analysis and Discovery
What sort of information do I need to include on my nutrition panel?
Your Nutrition Facts table needs to include all food ingredients, minerals, caloric value and typical serving size. Chemical analysis testing firms, specializing in finding nutritional values of food products, may guide you further.
Any and all allergens within the “top eight” must be clearly identified, and marked, in compliance with the Food Allergen Labeling and Consumer Protection Act of 2004. These allergens include: milk, eggs, fish, crustacean shellfish, tree nuts, peanuts, wheat, and soybeans.
Which is the most accurate form of nutrient testing?
Extensive laboratory testing and compared database measurements are recommended to achieve the greatest accuracy.
What kinds of laboratory testing may be used to find nutrition facts?
Instrumental-testing techniques can include: gas chromatography (GC), gas chromatography–mass spectrometry (GC-MS), high-performance liquid chromatography (HPLC), inductively coupled plasma mass spectrometry (ICP-MS) and many more.
How to Design and Create the Label
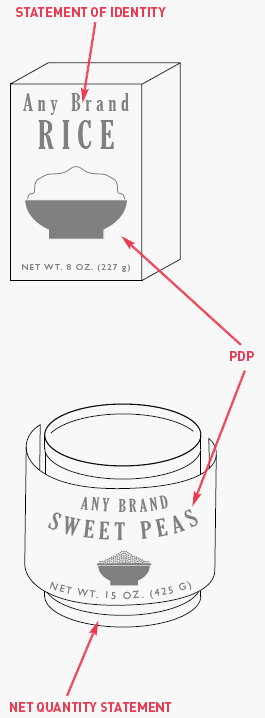
Where should the Nutrition Facts appear on food labels?
There are several ways to label packages and containers:
The Nutrition Facts may be presented on any label panel when the total surface available for labeling is 40 (or less than 40) square inches. Packages with more than 40 square inches of available space must place the nutrition information on either the Principal Display Panel (PDP) or information panel. The information panel is the panel immediately to the right of the PDP as seen by the customer facing the product. If there is insufficient space, the Nutrition Facts may be placed on any panel that may be easily seen by consumers.
Wait. What are the PDP and the alternate PDP?
The PDP is the portion of the package label that is “most likely to be seen” by the consumer at the time of purchase. Many containers are designed with two or more different surfaces that are suitable for display as the PDP. These are called “alternate PDPs”.
What are the minimum type sizes and other format requirements for the nutrition labels?
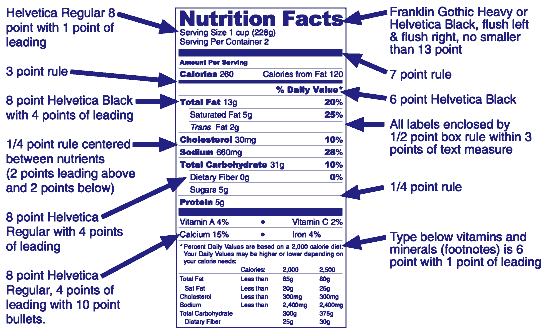
The Nutrition Facts label is typically boxed with black and printed on a white or neutral background. Other colors may be used as long as it is just one color. Anything listed under vitamins and minerals must be set in 6 point Helvetica Regular font with 1 point of leading. The illustration below indicates an example of the graphics that the FDA uses to display the Nutrition Facts label.
Typeface and Size
- The Nutrition Facts label uses 6 point or larger Helvetica Black and/or Helvetica Regular type. In order to fit some formats, the typography may be kerned as much as -4. Any tighter kerning will reduce legibility.
- Key nutrients and their percentage of Daily Value are set in 8 point Helvetica Black (but “%” is set in Helvetica Regular).
- Nutrition Facts is set in either Franklin Gothic Heavy or Helvetica Black to fit the width of the label flush left and flush right.
- Serving Size and Servings per container are set in 8 point Helvetica Regular with 1 point of leading.
- The table labels (for example, “Amount per Serving”) are set in 6 point Helvetica Black.
- Absolute measures of nutrient content (for example, “1g”) and nutrient subgroups are set in 8 point Helvetica Regular with 4 points of leading.
- Vitamins and minerals are set in 8 point Helvetica Regular, with 4 points of leading, separated by 10 point bullets.
What can be done if the regular Nutrition Facts label (i.e., the vertical format) does not fit the package?
On packages with more than 40 square inches available for labeling, the “side-by-side” format may be used if the regular Nutrition Facts label does not fit. In this format, the bottom part of the Nutrition Facts label (following the vitamin and mineral information) is placed directly to the right and separated with a line. If your label is not large enough to list additional vitamins and minerals after iron, you are allowed to list the additional nutritional statements to the right with a line that sets them apart from the footnotes.
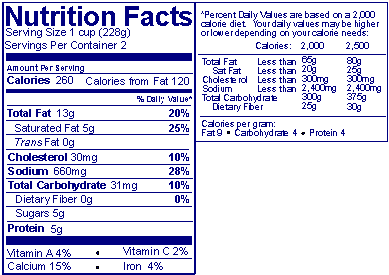
If the package has insufficient continuous vertical space (i.e., about 3 inches), another acceptable format is the “tabular” horizontal display.

A package design firm asked about the option of reversing the Nutrition Facts label copy as white type out of a dark-colored background on the grounds that reverse copy, with the appropriate size and color contrast, can be as readable as positive type.
According to FDA guidelines, the nutrition information “shall be all black or one color type, printed on a white or another neutral contrasting background whenever practical.” Reverse printing or the use of other colors is permitted as long as there is no impairment in readability. If there is, your label must compensate with the use of other graphics techniques, such as increased type size. Reverse printing is not permitted as a form of highlighting (e.g. using reverse printing for only certain parts of the table) because it would interfere with the consistent look of the label.
Can we use a continuous print label that would result in the Nutrition Facts label being cut off at an odd spot, with the bottom of the label at the top of the package, and the top of the label near the bottom?
The FDA firmly says no. However, a continuous print label that includes one uncut Nutrition Facts label is acceptable.
Can the Nutrition Facts panel be printed on a label and affixed to a package (like a sticker)?
Yes, just as long as the label adheres to the product under the intended storage conditions (such as heat, cold, moisture, etc.). The label must be guaranteed to stick to the package upon point of sale.
On nutrition labels with more than one language, can nutrition information be provided in one bilingual Nutrition Facts label?
Yes. The nutrition information may be presented in two ways: separate nutrition labels for each language or one label. If you choose only one label, then the first language must be in English followed by the second language translating all required information. There is no need to repeat numeric characters if they are the same in both languages.
I have tried all the available format options, but without some modifications, I cannot make them work on my label. What can I do?
The FDA may permit alternative resources of compliance or additional exemptions to deal with special situations. If you need special allowances, please make your request in writing to:
Office of Nutrition, Labeling, and Dietary Supplements
HFS-800, 5100 Paint Branch Pkwy.
College Park, MD 20740
The letter should:
- Specify that you are requesting an exemption or special provision under 21 CFR 101.9(g)(9).
- Identify the particular product(s) that are the subject of the request.
- State the reason(s) why it is technologically infeasible or impracticable to adhere to the regulations for such products.
- Identify the proposed alternative procedure.
- If possible, include an example of the proposed label(s).
Is it permissible to use stickers to make changes in labeling?
Correcting label mistakes in any manner is acceptable as long as the final label is correct and complies with all regulations at the time of retail sale. The stickers should not cover other mandatory labeling and should strictly follow the prescribed guidelines on nutrition labeling.
Legal, Liability and Exemptions
If a company produces $51,000 worth of food, but had a total gross sales for all products, food and non-food, of $490,000, do they need to have a nutrition label?
No, the company is exempt as long as no nutritional claims are made. A company whose total gross sales for all products, food and non-food is $501,000, with only $49,000 of this figure representing sales of food, is also exempt. Under the Nutrition Labeling and Education Act, firms who have an annual gross sales made or business done in sales to consumers that is not more than $500,000 or have annual gross sales made or business done in sales of food to consumers of not more than $50,000 are exempt. The following chart illustrates the exemption:
SALES IN FOOD | TOTAL SALES (FOOD & NON-FOOD) | STATUS |
$50,000 or less | $500,000 or less | EXEMPT |
$50,000 or less | $500,001 or more | EXEMPT |
$50,001 or more | $500,000 or less | EXEMPT |
$50,001 or more | $500,001 or more | NOT EXEMPT |
What is the exemption for small food packages?
Small package that have less than 12 square inches total surface area available for labeling may print a telephone number or an address to obtain nutrition information. This exemption (using a telephone number or address in place of the Nutrition Facts label) is permitted only if no nutritional statement claims are made or nutrition information does not appear on the product label or in labeling and advertising.
If we label the nutrition in good faith, will FDA take legal action involving small mistakes?
FDA is unlikely to take regulatory action for minor errors. However, it is in your best interest, from a legal and reputation standpoint, to correct such errors during the next printing of labels.
So, what’s all the big fuss? Why all of these guidelines? What is the FDA most concerned with on my nutrition label?
The FDA is concerned about ingredients and allergens. All ingredients must be on the label unless you get an approved exemption (e.g. to protect trade secrets). Ingredients are listed in order of predominance, with the ingredients used in the greatest amount first and followed in descending order by those in smaller amounts.
All of the “top eight” allergens must be listed clearly if your product contains any of them. This includes: milk, eggs, fish, crustacean shellfish, tree nuts, peanuts, wheat, and soybeans. No exemptions.
Non-compliance with the Food Allergen Labeling and Consumer Protection Act (FALCPA) may result in civil sanctions, criminal penalties, or both under the Federal Food, Drug, and Cosmetic Act if one of the company’s packaged food products does not comply with the FALCPA labeling requirements.
My product may come into contact with other ingredients, possibly allergens.
The FALCPA understands that inadvertent cross-contamination can occur within even good manufacturing conditions. However, it is highly recommended that you describe what known ingredients may have come into exposure with your products, including the “top eight” allergens.
Referrals and Citations: For More Information
Where might I find more information about FDA guidelines on food labels?
The above material was compiled from the following links at www.FDA.gov, which provides complete and comprehensive materials in regards to their standards:
- Food Labeling Guide
- Chapter 3. General Food Labeling Requirements
- Chapter 7. Nutrition Labeling; Questions G1 through P8
- Guidance for Industry: Questions and Answers Regarding Food Allergens, including the Food Allergen Labeling and Consumer Protection Act of 2004
- 21 Code of Federal Regulations 101
Do you know of any chemical analysis firms or labs that can help me?
While Consolidated Label has not worked with, and cannot endorse any 3rd party company, the following few companies are examples of chemical laboratories specializing in food analysis:
- AOAC International – a non-profit scientific association.
- Intertek – global inspection, product testing and certification company based in London, UK.
- Medallion Labs – the analytical testing and consulting service of General Mills’ James Ford Bell Technical Center.
Is there a place where I can compare the results of my chemical analysis to other foods?
Yes. The U.S. Department of Agriculture created, and now operates, the National Nutrient Database for Standard Reference, a database of nutritional content for many generic and branded food products.
USDA:
- USDA Nutrient Database: http://www.nal.usda.gov/fnic/foodcomp/search/